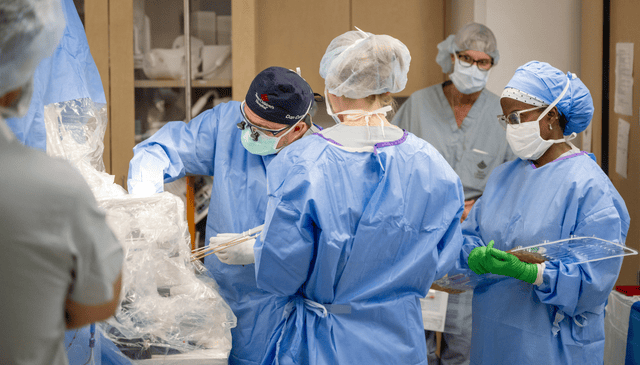
Texas Children’s is pleased to announce that a three-year-old girl has been successfully treated with the first-ever FDA-approved gene therapy treatment for AADC deficiency.
Aromatic l-amino acid decarboxylase (AADC) deficiency is an extremely rare, inherited neurological disorder that prevents the brain from producing dopamine and serotonin — essential chemicals for controlling movement, mood and basic nervous system functions. The literature reports approximately 350 people with this condition worldwide. Historically, there was no cure or approved treatment for AADC, and the shortened life expectancy was estimated between five and seven years of age.
“Texas Children’s was the largest contributor to the clinical trial in the U.S. that led to this drug’s approval,” said Dr. Daniel J. Curry, who performed the six-hour surgery. Dr. Curry is Director of Functional Neurosurgery and Epilepsy Surgery at Texas Children’s Hospital and Professor of Neurological Surgery at Baylor College of Medicine. “Before now, AADC deficiency was a hopeless diagnosis. With this treatment, we’ve entered a whole new era where we can deliver solutions to formerly untreatable genetic problems. This is the first step in hopefully many future strides toward the molecular correction of inborn deficits for which there used to be no cure.”
Click here to read the press release.

