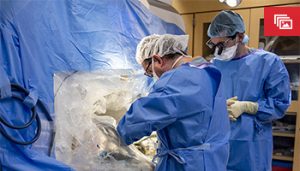
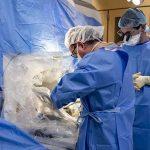
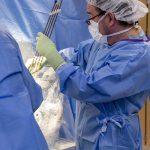
In a clinical trial aimed at assessing the method of delivery of an experimental therapy for aromatic L-amino acid decarboxylase (AADC) deficiency, neurosurgeon Dr. Daniel Curry performed the first intracranial gene therapy procedure at Texas Children’s. Dr. Curry serves as Texas Children’s Director of the Functional Neurosurgery and Epilepsy Surgery program and he holds the John S. Dunn Foundation Endowed Chair for Minimally Invasive Epilepsy Surgery.
The gene therapy from PTC Therapeutics, PTC-AADC (eladocagene exuparvovec), is being developed for the treatment of AADC deficiency, a rare genetic disorder where children are not able to make dopamine and serotonin in their bodies. Children with AADC deficiency have developmental disabilities and have no ability to control their muscles. Without basic motor functions, these children are not able to develop motor milestones of crawling, sitting, standing or walking and have an average lifespan of only 10 years. Comfort care has been the only treatment for children with AADC.
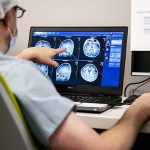
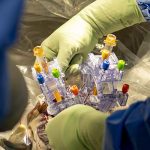
Data collected from previous clinical trials of PTC-AADC demonstrated durable and meaningful clinical effects, as well as a favorable safety profile to participants. The current study assesses the use of the SmartFlow MR-compatible ventricular cannula to deliver the therapy directly to the putamen, the area of the brain that controls movement.
Dr. Curry is a principal investigator on “A Study of SmartFlow® Magnetic Resonance (MR) Compatible Ventricular Cannula for Administering Eladocagene Exuparvovec to Pediatric Participants.” There are currently seven sites for the clinical trial. Several patients have been treated and more are approved for enrollment at Texas Children’s.
Many Texas Children’s service areas are involved in supporting patients and families during this study including co-investigators from Neurology Dr. Lisa Emrick, Dr. Mariam Hull and Dr. Mered Parnes; senior researcher Sarah Wisor-Martinez from Neurosurgery; Dr. Andy Sher and Rodney Colon from Radiology; Huyen Reardon from Nuclear Radiology; from Physical Therapy Dr. Katie Robey and Dr. Kati-Lyn Tierney; and Dr. Nick Carling, Dr. Steve Stayer and Dr. Megha Kanjia from Anesthesiology. Initial results from the study are anticipated in late 2022.